One of the most common questions asked by homeowners about water softeners is why softened water feels the way it does. The term soft water refers to water that is stripped of the hard water or scale forming ions like calcium and magnesium. In order to understand the effects soft water has on aesthetics and cleaning, we need to understand the core concepts of surface tension, and the kinetics of soap and soft water.
SURFACE TENSION
Surface tension is the tendency of a liquid to form a relatively tough "skin" or film on its surface. Surface tension is caused by the attraction between the molecules of a liquid, and it is surface tension that causes water molecules to stick together and form drops. The greater the surface tension, the greater the ability to resist externals force. Water molecules cling to one another through the force known as hydrogen bonding. The hydrogen bonding is what gives water its high surface tension, which we observe when water beads on a surface or why small insects skate across the surface of a pond. The pull between water molecules is strong and creates a net neutral force. A single molecule in a droplet is surrounded on all sides by other water molecules. Water molecules on the surface bond more aggressively to one another because they are forced into contact with molecules in the air and this is what gives water its "film" on the surface. Water droplets form on surfaces because of a stronger pull by water molecules attraction to one another, which in turn creates less work to form a sphere than to form a flat film. Lowering the surface tension makes water "wetter" by decreasing its resistance to compression. A good example is the act of pushing on a droplet of water with your finger. As you apply force to the droplet, the surface tension is overcome until a flat film has formed. On a porous surface, having a lower surface tension allows water to penetrate deeper allowing for better cleaning. The addition of soap or the use of hot water will both lower the surface tension of water.
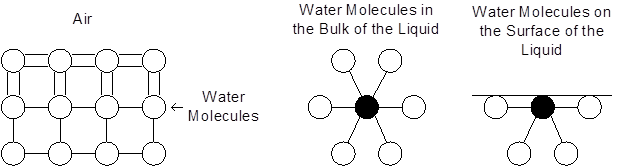
The image shows the interactions between water molecules in a liquid and the interaction of water molecules at the surface. Water molecules at the surface that are in contact with the air have stronger bonds than those molecules that are in the bulk of liquid.
It is well known that water softeners lower the surface tension of water and that soft water aids in cleaning as a result, but why? Water softeners function through the process of ion exchange, i.e. exchange calcium and magnesium ions for sodium ions. The conclusion can be drawn that sodium lowers the surface tension of water while calcium and magnesium ions increase the surface tension. A deeper look into the physical properties of these ions reveals this unique phenomenon. The impact on surface tension is partially a result of the valence, or charge of the ions. The valence of an ion is a measure of its ability to combine with other atoms when it forms chemical compounds or molecules. The higher the valence of the cations is, the greater the pull towards the bulk of the liquid will be, and thus, the greater the surface tension. Calcium and magnesium both have a higher valence (+2) than that of sodium (+1). There are other factors that influence the "wetness" or "slippery" feel of soft water including pH and alkalinity. Typically the higher the alkalinity and pH, the greater the impact of this phenomenon. This may help to explain why naturally soft water or reverse osmosis water do not have the same "wetness" or "slippery" feel.
The Kinetics of Soap
Natural soaps are comprised of water-soluble sodium or potassium salts or fatty acids, while they are produced commercially by combining a strong alkali with processed animal or vegetable fats. The specific alkali used is what differentiates the soaps between sodium stearate based soaps (hard soaps) and potassium stearate based soaps (soft soaps). Soap molecules are comprised of a carboxylate or hydrophilic end that is attracted to water molecules and a hydrocarbon chain or hydrophobic tail that is repelled by water molecules. The hydrophobic end will not mix with water and therefore works its way to the surface being cleaned and forces its way underneath contaminants on said surface. The opposing attraction of the head and tail of the soap molecule results in the lifting and suspension of contaminants within the water molecule structure. In lay terms, the hydrophobic end grabs the dirt and the hydrophilic end grabs onto the water and is shown visually below.

The image displays the kinetics of soap on dirt on a surface. Moving from left to right, the dirt is removed from the surface as the hydrophobic ends (tail) works its way to the surface and the hydrophilic end (head) is attracted to surrounding water molecules.
A key function of soap is that it lowers the surface tension of water, allowing the water to make more uniform contact with the surface being cleaned and penetrate porous surfaces. Using soft water increases the cleaning abilities of soap and also prevents the stearates from forming insoluble precipitates of calcium and magnesium stearates, also known as soap scum. This is what causes soap rings in showers and fabrics feeling stiff when washed with hard water. The higher the hardness, the more soap that will be used. One of the benefits of soft water is that less soap is required for normal cleaning, showering, or laundering.
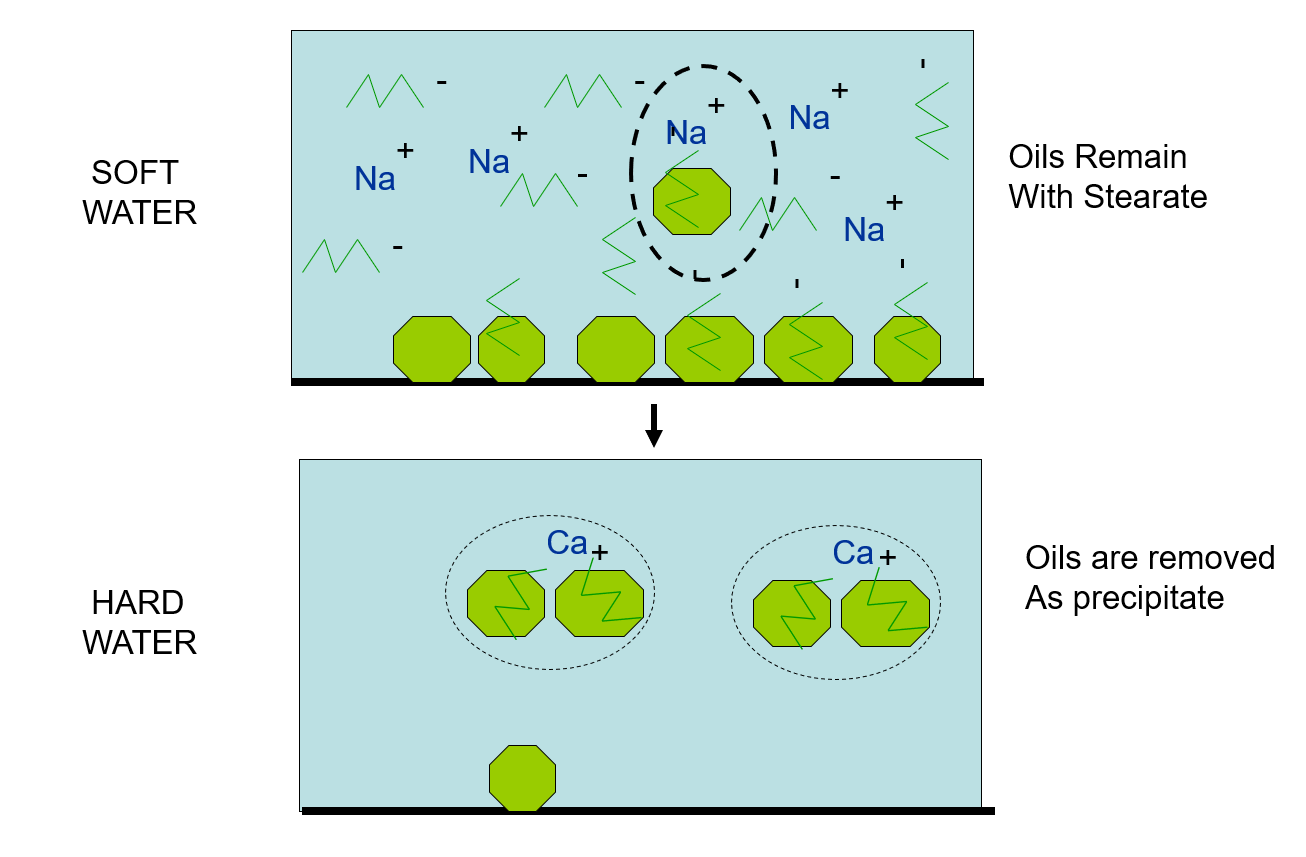
In soft water, oils are removed from the surface by the sodium stearate, but remain dissolved in solution. In hard water, the calcium stearate combines with oils and precipitates out of solution to form curds or soap scum.
AESTHETICS OF SOFT WATER
The aesthetics and feel of soft water is all based on the individual perceptions and expectations of the end user. Certain descriptive terminology have both positive and negative connotations depending on the individual. Soft water itself (both naturally occurring and mechanically conditioned) can feel more slippery than hard water. People use terms like slippery, silky, slimy, squeaky, etc. when describing how any water feels, whether the implication is positive or negative. For this reason, the best we can do is clearly identify the reasoning behind why these sensations occur, regardless of how they are interpreted. Due to the lower surface tension created by softening through ion exchange, there is an increased "wetting" effect of soft water even in the absence of soap. Reduced surface tension allows it to wet the skin, better forming a thin, low friction layer on the surface of the skin. The sensation of "slipperiness" is essentially caused by low friction between two surfaces. Depending on the individuals perception of this feeling, they may describe it using positive descriptors like silky for soft water or more negative descriptors like slimy. Depending on the individual's perception, we can install bleed valves and mix raw with treated water to control the level of hardness through the treatment system. Some people may prefer the trade off of not completely softened water for a reduction in the wetness of the treated water. The best we can do is educate our customers on the benefits of soft water, provide good explanations as to why they experience what they are experiencing, and how we can best meet or adapt to the individual needs of the end user.