Any water analysis report will provide the standard information including pH, hardness, alkalinity, and TDS. TDS or Total Dissolved Solids is somewhat underappreciated and overlooked in terms of water chemistry and treatment. Total Dissolved Solids is defined as the total weight of solids that are dissolved in the water, given in ppm per unit volume of water, often expressed as milligrams per liter or mg/L. TDS is determined by filtering a given volume of water (usually through a 0.45 micron filter paper), evaporating it at a defined temperature in a drying oven (103-105 °C), and then weighing the residue. The other, more common option is using a TDS or conductivity meter that measures the electrical conductivity of the water. This method provides an estimate of the TDS present, as conductivity is not precisely proportional to the weight of an ion, and non conductive substances cannot be measured by electrical tests. TDS can tell a lot about water chemistry while contributing to how it tastes and feels.
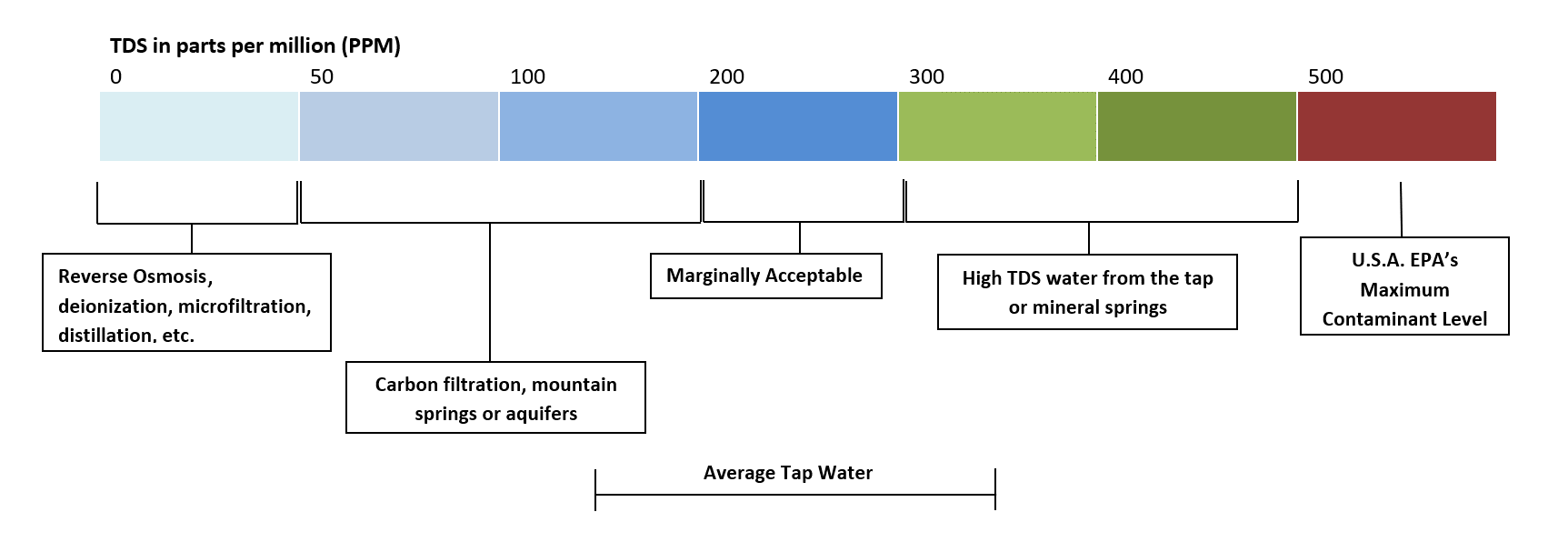
TDS is a product of the aquifer media that the groundwater passes through before it enters a water well. Common cations that contribute to TDS are calcium, magnesium, potassium, and sodium. Common anions include bicarbonate, carbonate, chloride, nitrate, sulfates, and silicates. We often recommend that dealers and installers carry a TDS meter with them in the field for on site analysis. Naturally soft well water typically has a low TDS, 50-100 mg/L while sea/brackish water samples have an extremely high TDS at about 35,000 mg/L. The National Secondary Drinking Water Standard for TDS in the United States is 500 mg/L.
Naturally low TDS water can often be associated with a low pH and low alkalinity. Naturally low TDS water tends to be more aggressive and cause corrosion issues. Due to the low dissolved solids and lack of alkalinity, it is not uncommon on water with low TDS (below 50 ppm) and a neutral pH to exhibit corrosive tendencies because the water has a greater affinity to dissolve or "attack" plumbing fixtures or solder on copper plumbing. Water is known as the universal solvent, so it makes sense that it if there is little or no solids in the water to begin with, that it will have an affinity to dissolve or attack plumbing systems. During pH correction practices, the TDS is increased by the dissolution of minerals (calcite or magnesium oxide). In the absence of a pH probe or test strips, a TDS meter can help determine if a neutralizer is working or if the unit may need to be recharged. The total increase in TDS after water passes through a neutralizer can be calculated if the influent pH, CO2 concentration, and total alkalinity are known. Low TDS water tends to be better tasting and is extremely important in the food and beverage industry. From brewing coffee and beer to making bread, industry professionals are always aware of TDS levels. Often times in these industries, water is stripped of its mineral content through distillation or reverse osmosis, then remineralized with a specific mineral profile to achieve the desired TDS and flavor profile.
High TDS can cause issues and concerns as well. Private well owners should be concerned if the TDS of their well supply reaches 500 mg/L or higher. High TDS water can cause staining of fixtures, and leave heavy residue or scale after evaporating. Often times, some form of surface contamination or runoff from road salt applications is a likely contributor. As the water table and precipitation patterns changes seasonally, groundwater sources can see fluxes in the TDS. Well water sources that are coastal tend to see high TDS waters because of the intrusion of sea water into their groundwater supply. The contamination of well water from road salt or sea water will also contribute higher concentrations of chlorides, which can create serious corrosion issues in plumbing systems. Well water can also have naturally high TDS if the groundwater passes through limestone or other high carbonate formations. High TDS water can have an undesirable flavor that is salty or bitter depending on its composition. Generally, we recommend Point of Use Reverse Osmosis systems for TDS levels equal to or greater than 500 mg/L. In pH correction applications, the TDS can increase significantly if the water is very acidic, or the free CO2 concentration is high. Along the same lines, different pH correction minerals will contribute differently to TDS increase, and their effectiveness may vary depending on the TDS of the raw water. Generally, the higher the TDS of an acidic water is, the more likely that a stronger neutralizing media will be needed.